Abstract
Background:
Carfilzomib (CFZ) is a potent, irreversible proteasome inhibitor (PI) licenced in patients with multiple myeloma (MM) demonstrating improved progression free and overall survival (OS) to standard of care therapies. However, CFZ is also associated with hypertension (HTN) and rarely cardiac toxicity. The exact mechanism is unclear but may be due to a disturbance of endothelial nitric oxide synthase and nitric oxide. Incidence of HTN in a pooled analysis of CFZ trials (Chari et al, Blood 2018, n=2044) showed all grade (G): 18.5%, ≥G3 5.9%. There is less data in the real world setting.
Methods:
This was a single centre retrospective analysis of all patients treated with CFZ between 2015-2018. BP was recorded in routine nursing records prior to each CFZ infusion in triplicate at each visit 10 minutes apart and the median recorded. HTN was graded by CTCAE criteria V4 (note: G1=pre-HTN (120-139/ 80-89)) and pulmonary hypertension as mean pulmonary arterial pressure (mPAsp) >25mmHg (American College of Cardiology criteria). Baseline demographics were obtained from medical records. OS was estimated using Kaplan Meier Curves and correlative analysis by Cox regression models.
Results:
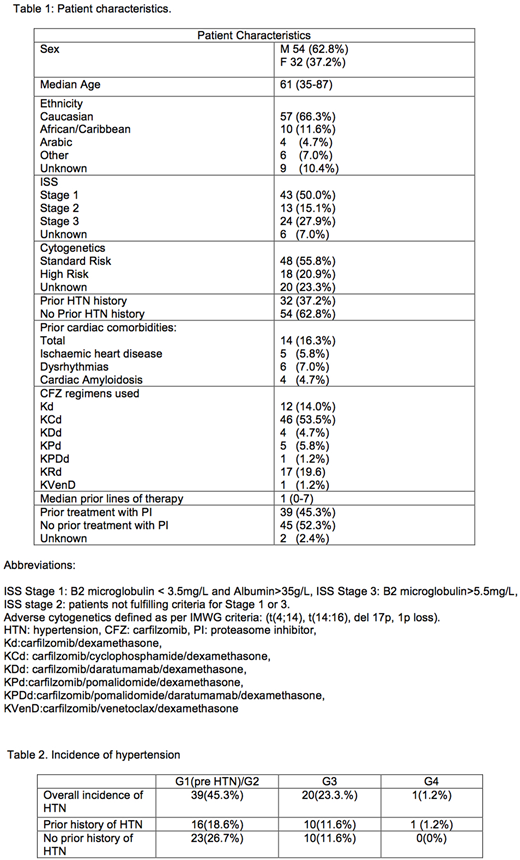
86 patients and 1976 consecutive BP recordings were evaluated with a mean of 23 BP assessments per patient (1-74). Demographics are shown in Table 1. 32 patients (37.2%) had prior history of HTN and 14 (16.3%) had prior cardiac co-morbidity (ischaemic heart disease, dysrhythmias and cardiac amyloidosis). Initial dosing of CFZ was 20mg/m2, increasing to 27mg/m2 (n=30 (34.9%)), 36mg/m2 (n=18 (20.9%)), 45mg/m2 (n=2 (2.3%)), 56mg/m2 (n=35 (40.7%)), 70mg/m2 weekly (n=1(1.2%)). Median time on therapy was 5.3 months (0-26) with a median of 6 (1-27) cycles.
The overall incidence of all grade HTN was 60 (69.8%), predominantly G1-2 and was similar in those with and without pre-existing HTN (Chi squared test p=0.4) (Table 2). 11(13%) required intervention with anti-HTN medications for ≥G2 HTN which then returned the BP to baseline. Those treated at ≥45mg/m2 of CFZ had more episodes of HTN compared to those treated at 27-36mg/m2 (OR 3.65, 95% CI: 1.39-9.21, p<0.01) despite similar co-morbidities per group. Age >65 years was not associated with increased risk of HTN (OR 1.32, 95% CI 0.45-3.73, p=0.63) nor was ethnicity (Chi squared test p=0.1). 25 patients required treatment interruption for any cause, of which 12 were due to HTN (median 7 days (1-23)).13 patients required dose reduction for any cause, and 9(10%) required reductions due to HTN, mainly at 56mg/m2 (27 n=1(1.2%), 36 n=2(2.3%), 56 n=7(8.1%)).
The planned median cumulative dose for the number of cycles received was 1264mg/m2 overall (36-5772) however, the actual median cumulative dose delivered was 784 mg/m2 (36-3248). The difference was the greatest with higher carfilzomib doses (≥56mg/m2 vs 27-45mg/m2 (Chi squared test, p<0.01)). Planned vs actual dose delivered for number of cycles of CFZ received was: 27mg/m2, 634 vs 472; 36mg/m2, 1219 vs 722; 45mg/m2, 785 vs 744; 56mg/m2, 2514 vs 1282; 70mg/m2, 1210 vs 770.
15(17%) patients developed cardiac complications including pulmonary HTN n=10(12%) and cardiac failure n=8(9%). Unlike HTN, cardiac complications were not associated with CFZ dose (<36mg/m2 vs ≥36mg/m2 OR 1.2, 95% CI 0.42-3.51, p=0.75) and were not related to development of HTN (OR 2.40, 95% CI 0.80-6.60, p=0.12) or prior history of HTN (OR 1.79, 95%CI 0.53-5.50, p=0.35). 1(1.2%) death was observed in a patient due to cardiac failure and progressive disease.
OS was unaffected by HTN, cardiac toxicity, pulmonary HTN or HTN related treatment delays (median OS not reached regardless of developing cardiovascular adverse events, HR 1.45 95% CI 0.39-5.37, p=0.57; median follow up 17 vs 31 months respectively).
Conclusions: This real world data demonstrated a higher incidence of HTN compared to clinical trials, however most were low grade toxicities. This may be partly due to under-reporting of G1 events which may not be clinically significant. Dose reductions were more frequent at CFZ doses ≥45, leading to a reduction in total cumulative dose received. Close monitoring and early intervention for HTN is therefore required to prevent further complications and to maintain cumulative dosing. In this dataset, cardiac complications did not appear to be related to HTN.
Cheesman:Celltrion: Other: Speaker Fee; Roche: Other: Advisory board. Wechalekar:Janssen: Honoraria. Rabin:Janssen: Consultancy, Other: Travel support, Speakers Bureau; Takeda: Consultancy, Other: Travel support , Speakers Bureau; Celgene: Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau. Yong:Janssen: Honoraria, Speakers Bureau; Celgene: Honoraria; Takeda: Speakers Bureau; Amgen: Honoraria, Research Funding, Speakers Bureau. Popat:Amgen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal